Advanced stage ovarian cancer
Cytoreductive/ debulking surgery
The aim of the cytoreductive surgery or debulking surgery is to remove as much of the tumour as safely possible. Besides removing the female genital organs, the surgery may remove tissues from nearby organs, such as liver, bowel or spleen. The tissues will then be sent to laboratory to find out if the cancer has spread.
If the surgery can remove most or all the cancer, heated chemotherapy may be infused into the abdomen during the debulking surgery to kill any cancer cells in the peritoneum. This process is called Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Traditional intravenous chemotherapy may not be effective in treating cancer cells that have spread to the peritoneum. HIPEC has the advantage of single administration, and all the peritoneal surfaces are exposed to heated chemotherapy. The chemotherapy needed to be heated as this higher temperature can improve its effectiveness in killing the cancer cells.

If the tumour size is too big and has spread to the peritoneum, your doctor may prescribe 3 to 6 cycles of chemotherapy (neoadjuvant chemotherapy) to shrink the size of the tumour before operation.
Chemotherapy
Chemotherapy is recommended for all advanced stage ovarian cancer. Combination of platinum-based (carboplatin, cisplatin, oxaliplatin) and taxanes-based (paclitaxel, docetaxel, nab-paclitaxel) chemotherapies are mostly recommended. Paclitaxel-carboplatin is the preferred option for all stages. The chemotherapy is usually given once every 3 weeks, lasting for around 4 to 6 months.
Your doctor may add a targeted agent called bevacizumab on top of the chemotherapy. Bevacizumab is a targeted agent that stops blood vessel growth. Studies have shown that adding bevacizumab on top of chemotherapy would extend the progression-free survival (i.e. the length of time people with advanced ovarian cancer lived without their tumours growing or spreading compared with chemotherapy alone, 18.2 months vs. 12.0 months).
After chemotherapy, your doctor may consider giving maintenance therapy to reduce the chance of progression.
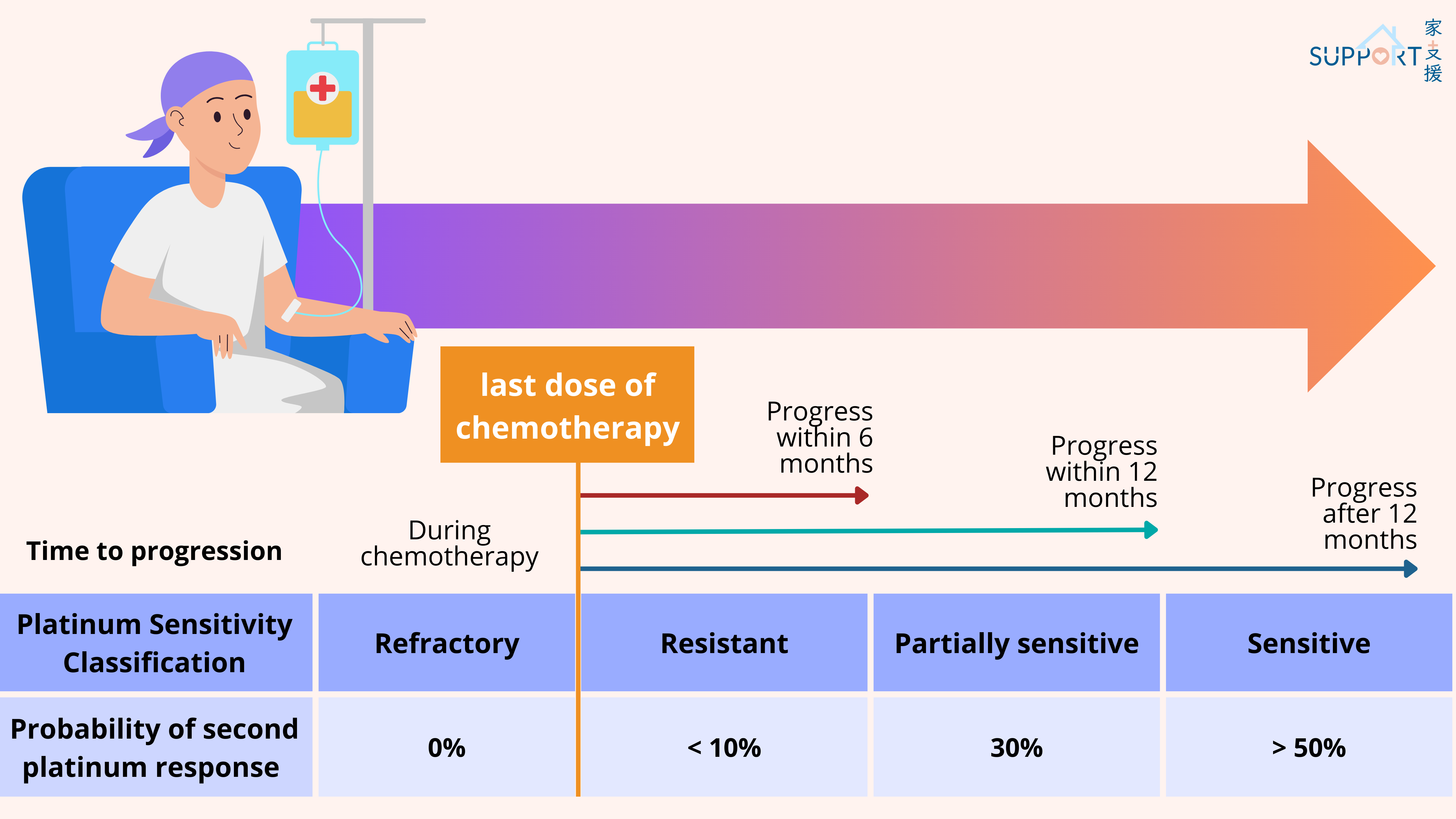
If the disease progresses unfortunately, your doctor will calculate the duration from the last chemotherapy to the time that the cancer progresses again. If it is over 6 months, you may be treated with platinum-based chemotherapy again. However, if less than 6 months (this is called platinum-refractory disease), other drug combinations may be used, including:
- pegylated liposomal doxorubicin
- topotecan
- trabectedin
- etoposide
- gemcitabine
Targeted therapy
Targeted therapy may be used together with chemotherapy after chemotherapy as maintenance therapy in advanced ovarian cancer. There are two main types of targeted therapies: Anti-VEGF inhibitor (bevacizumab) and PARP inhibitors.

1. Anti-VEGF inhibitor (bevacizumab)
- Bevacizumab is an antibody that binds vascular endothelial growth factor (VEGF) and prevents it from being active. VEGF promotes new blood vessels to form into the tumour and deliver nutrition to it. Anti-VEGF inhibitor will bind to VEGF and stop blood vessels from growing and can help starving the tumour. Studies have proven that combination of bevacizumab and chemotherapy can improve the progression-free survival in patients with stage III or stage IV ovarian cancer.
- Administration: intravenously, given every 3 weeks
- Common side effects: nausea, vomiting, diarrhoea, tiredness, headache, low cell counts, nosebleeds, high blood pressure, proteinuria
- Severe side effects (rare): thromboembolic events (DVT, pulmonary embolism, heart attack, stroke), heart failure, bowel perforation, severe bleeding, kidney damage
2. PARP inhibitors (Olaparib, niraparib, rucaparib)
- PARP is an enzyme that involves in repairing damaged DNA. By blocking this enzyme in cancer cells, the DNA inside the cancer cells will less likely be repaired, leading to cell death and slowing down tumour growth.
- Olaparib , niraparib and rucaparib are PARP inhibitors that can be used with or without bevacizumab for maintenance therapy for advanced ovarian cancer after first- or second-line platinum-based chemotherapy. The PARP inhibitors are more effective in patients with BRCA mutation or those without BRCA mutation but with homologous recombination deficiency (HRD).
- Administration: oral medication,
- Common side effects: fatigue, diarrhoea, increased risk of infection, bleeding, headache, dizziness, taste change, changes in liver or renal function
- Rare but severe side effects: myelodysplastic syndromes, acute myeloid leukaemia. Patients need to continue blood cell count monitoring even after stopping PARP inhibitors.
3. Mirvetuximab soravtansine (Elahere)
- Many ovarian cancers have high levels of the FR-alpha protein. Mirvetuximab soravtansine (Elahere) is an antibody-drug conjugate (ADC), which is a lab-made antibody linked to a chemotherapy drug. It targets this protein, delivering chemotherapy directly to cancer cells. It's used for epithelial ovarian cancer that tests positive for FR-alpha and resists platinum chemotherapy.
- Administration: intravenously, given every 3 weeks
- Common side effects: nausea and vomiting, diarrhoea or constipation, feeling tired, belly pain, low blood cell counts, and changes in mineral levels in the blood
- Rare but severe side effects: eye problems which can include blurred vision, dry eyes, light sensitivity, eye pain, or vision changes; serious lung disease; nerve damage leading to numbness, tingling, or weakness in the hands or feet
4. NTRK inhibitors (Larotrectinib, entrectinib)
- Some ovarian cancers have NTRK gene changes, causing abnormal cell growth. Larotrectinib and entrectinib target these changes. They help patients with advanced ovarian cancer with NTRK fusion and the tumors grow despite other treatments.
- Administration: oral medication, taken once or twice a day
- Common side effects: dizziness, fatigue, nausea, vomiting, constipation, weight gain, diarrhoea
- Rare but severe side effects: abnormal liver tests, heart problems, confusion
5. BRAF and MEK inhibitor combination (Dabrafenib, trametinib)
- Some ovarian cancers have BRAF V600E gene changes, causing abnormal cell growth. Dabrafenib and trametinib target these changes. They help patients with advanced ovarian cancer whose tumors grow despite other treatments.
- Administration: oral medication, taken once or twice a day
- Common side effects: fever, rash, headache, joint pain, cough, nausea, vomiting, diarrhoea, muscle pain, dry skin, decreased appetite, high blood pressure, and difficulty in breathing
6. RET inhibitor (Selpercatinib)
- In rare cases, ovarian cancer cells have RET gene mutations, leading to abnormal growth. Selpercatinib targets these proteins, aiding patients with advanced ovarian cancer resistant to other treatments.
- Administration: oral medication, taken twice a day
- Common side effects: oedema, diarrhoea, fatigue, dry mouth, increased blood pressure, abdominal pain, constipation, rash, nausea, headache
7. HER2 inhibitor (Trastuzumab deruxtecan)
- Occasionally, ovarian cancer cells exhibit HER2 gene changes, causing uncontrolled growth. Trastuzumab deruxtecan is an ADC that targets these proteins, benefiting patients with advanced ovarian cancer unresponsive to other therapies.
- Administration: intravenously, given every 3 weeks
- Common side effects: increased risk of infections and bleeding, nausea and vomiting, diarrhoea or constipation, loss of appetite, fever, feeling tired, hair loss
- Rare but severe side effects: lung disease (coughing, wheezing, trouble breathing, or fever), heart disease
Immunotherapy
Immunotherapy is not commonly used in metastatic ovarian cancer. Immune checkpoint inhibitors can be used for treating metastatic ovarian cancer if the tumour has high microsatellite instability (MSI-H), DNA mismatch repair deficiency (dMMR) or high tumour mutational burden (TMB-H). However, these mutations are very rare in ovarian cancer. Examples of immune checkpoint inhibitors for ovarian cancer include pembrolizumab and dostarlimab.
Administration: intravenously, given every 3 weeks
Common side effects: fatigue, cough, nausea, itching, skin rash, loss of appetite, constipation, joint pain, diarrhoea
Rare but severe side effects: infusion reactions that can include fever, chills, flushing of the face, rash, itchy skin, feeling dizzy, wheezing, and trouble breathing; autoimmune reactions which can cause serious or even life-threatening problems in the lungs, intestines, liver, hormone-making glands, kidneys, or other organs