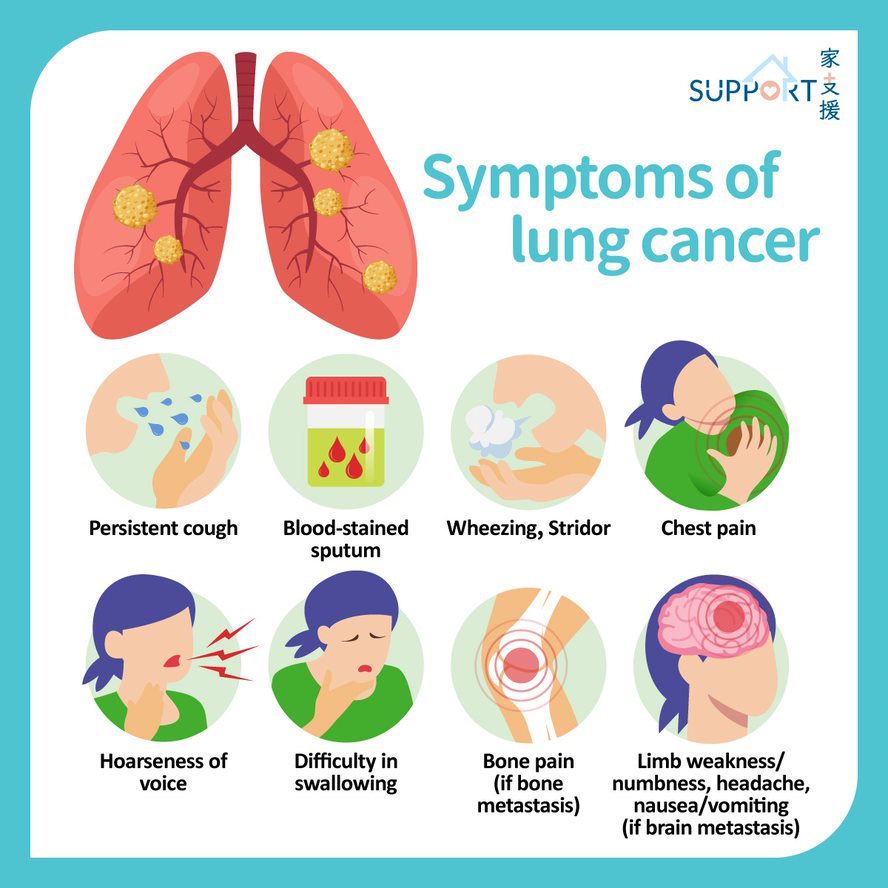
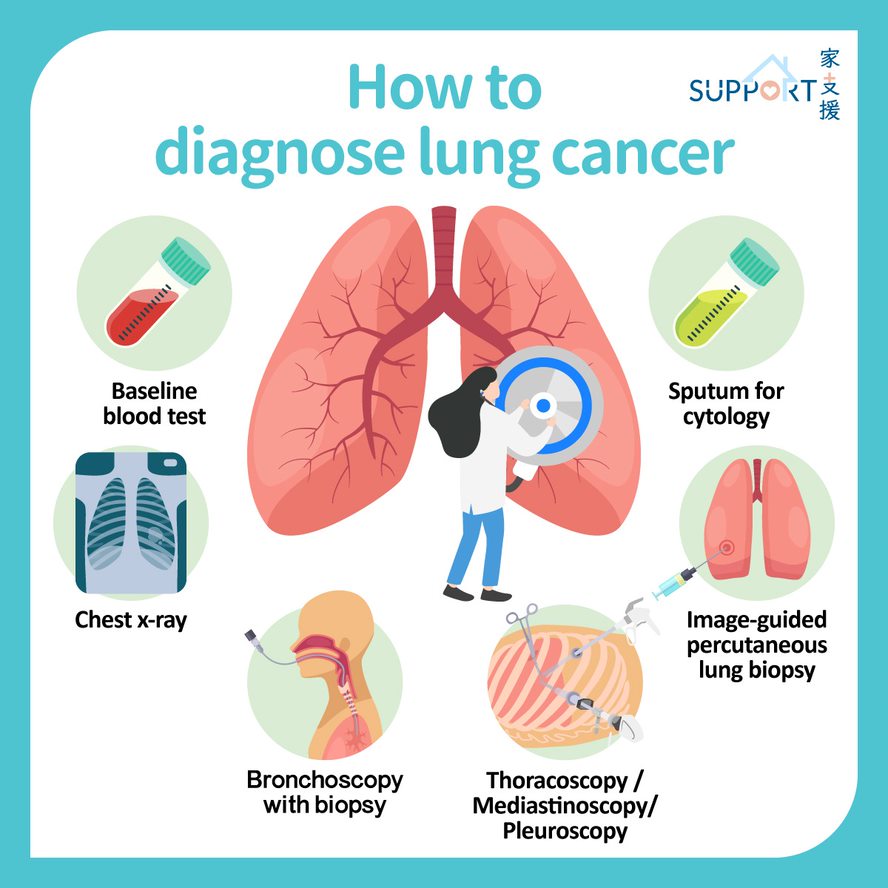
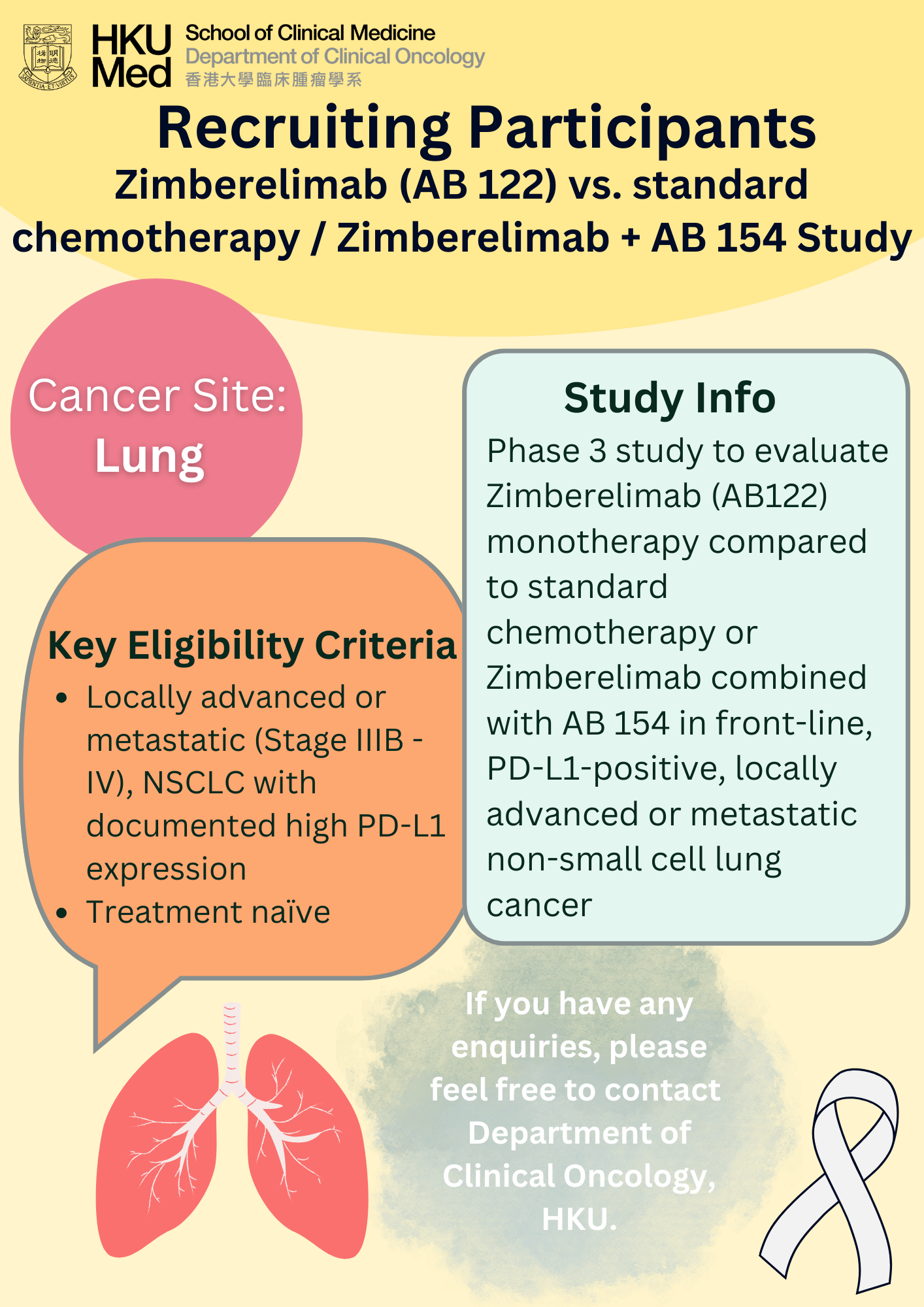
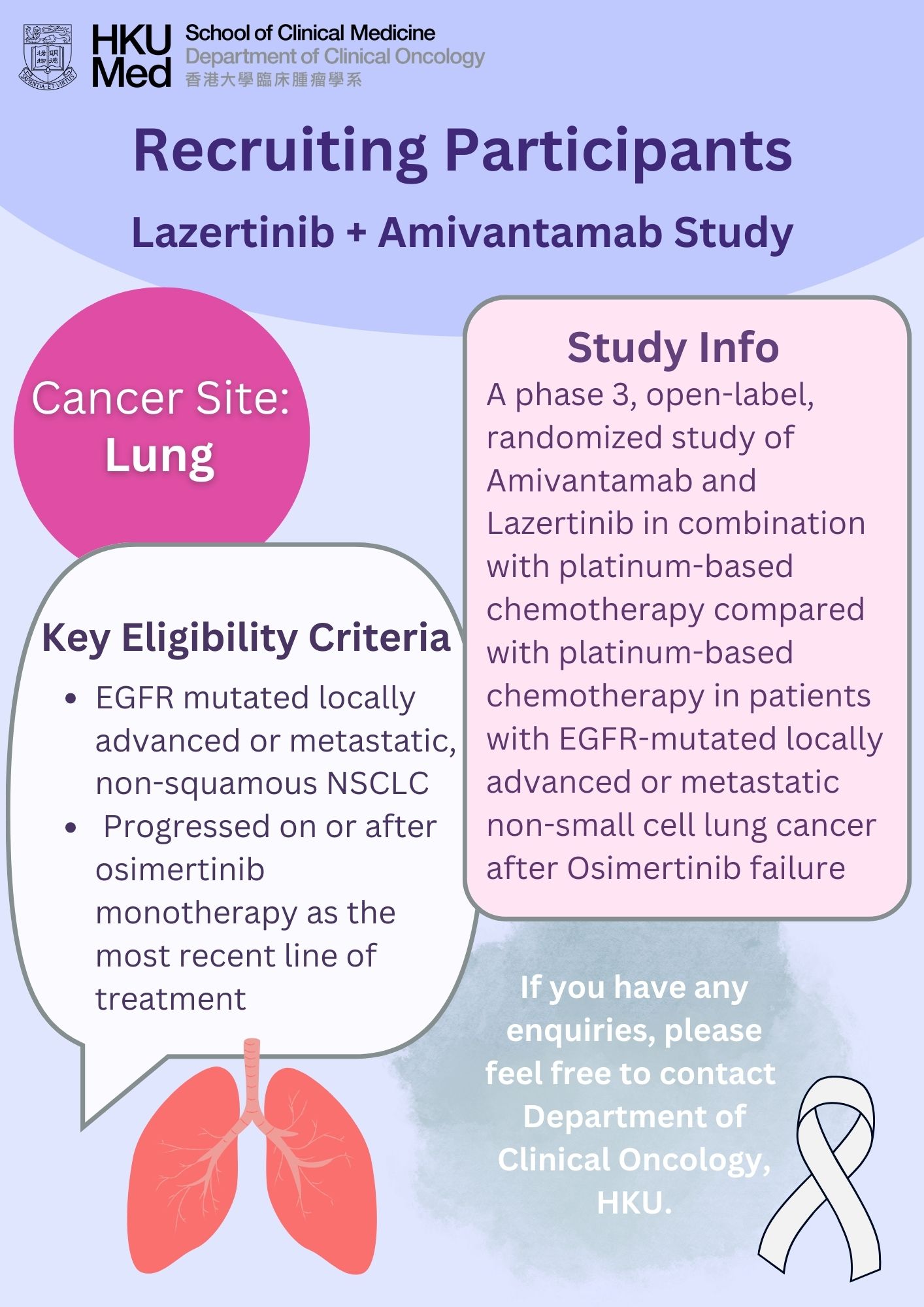
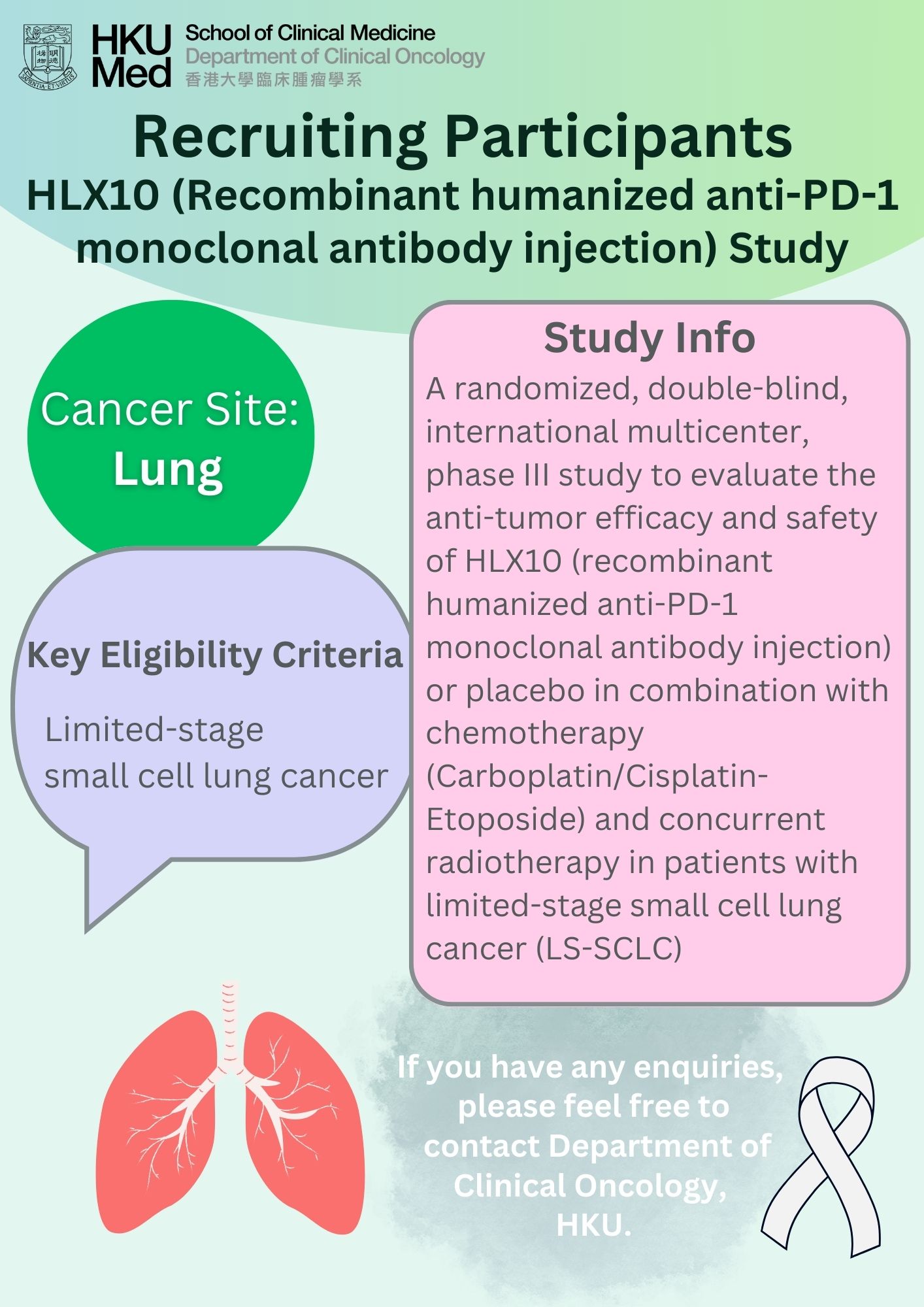
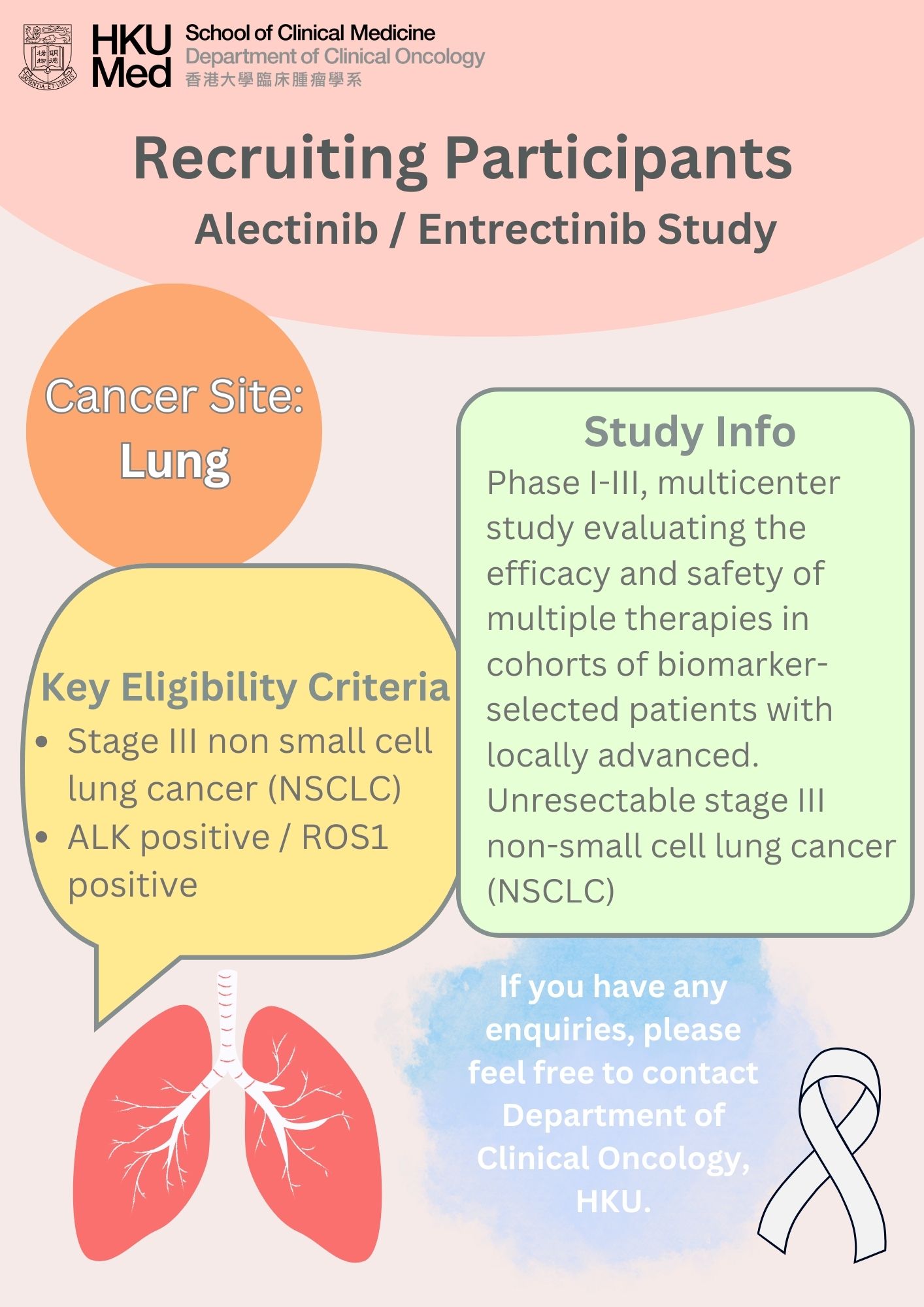
Lung cancer was the commonest cancer in Hong Kong. In 2023, there were 6,111 new cases of lung cancer in Hong Kong, accounting for 16.1% of all new cancer cases. Over the past 10 years (from 2014 to 2023), the number of new lung cancer cases rose by 30.4%. The crude annual incidence rate of lung cancer per 100,000 population was 81.1. The incidence was higher in male compared with female, with a male-to-female ratio of about 1.4 to 1. The median age at diagnosis was 70 in male and 68 in female.
Lung cancer was also the first leading cause of cancer deaths in Hong Kong. In 2023, a total of 3,880 people died from this cancer, accounting for 26.1% of all cancer deaths.
What is lung cancer?
The lungs are a pair of organs located in the chest. Lungs consist of the bronchus, bronchioles and air sacs known as alveoli. During inhalation, lungs take oxygen into the bloodstream and deliver it to the rest of the body. During exhalation, they remove carbon dioxide from the bloodstream.
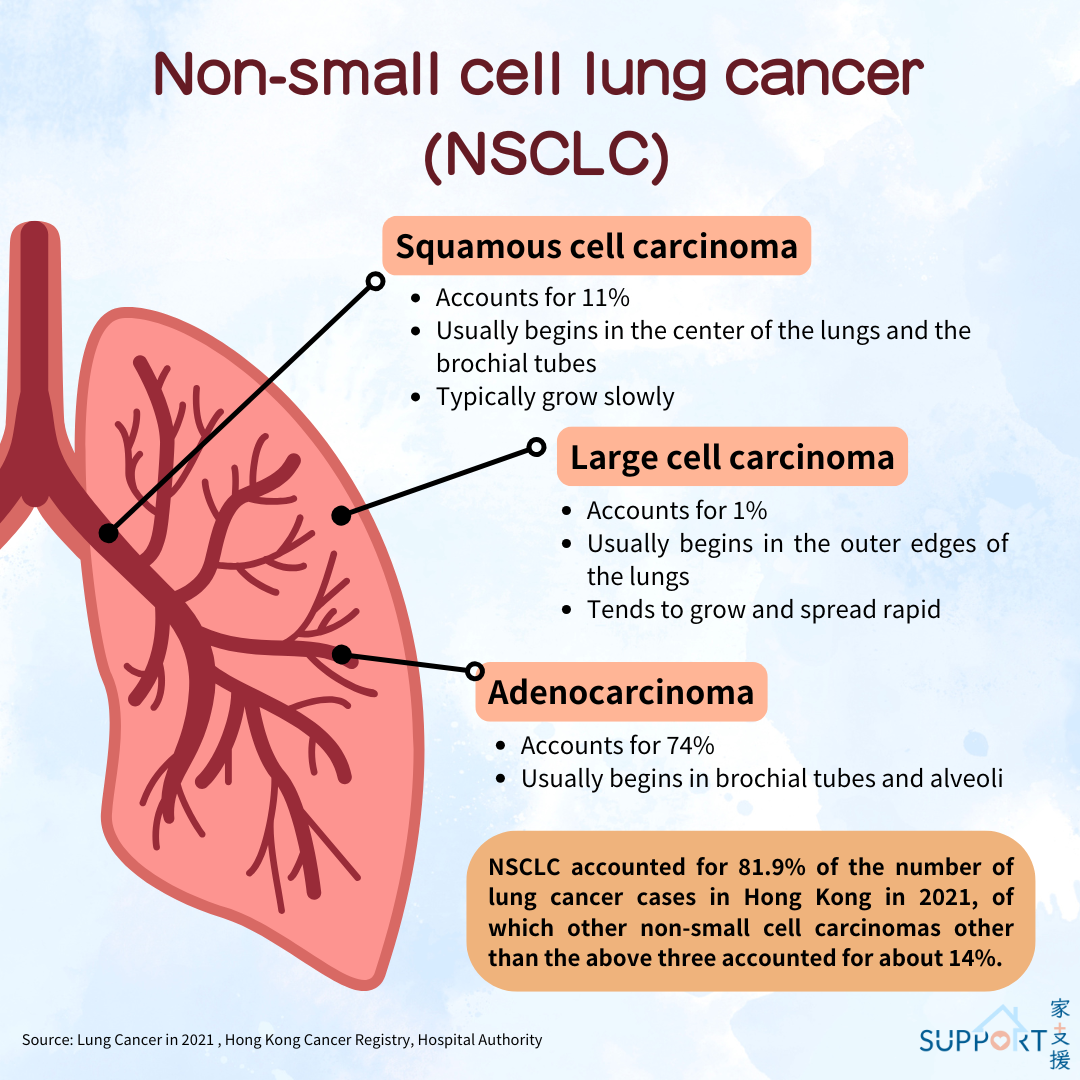
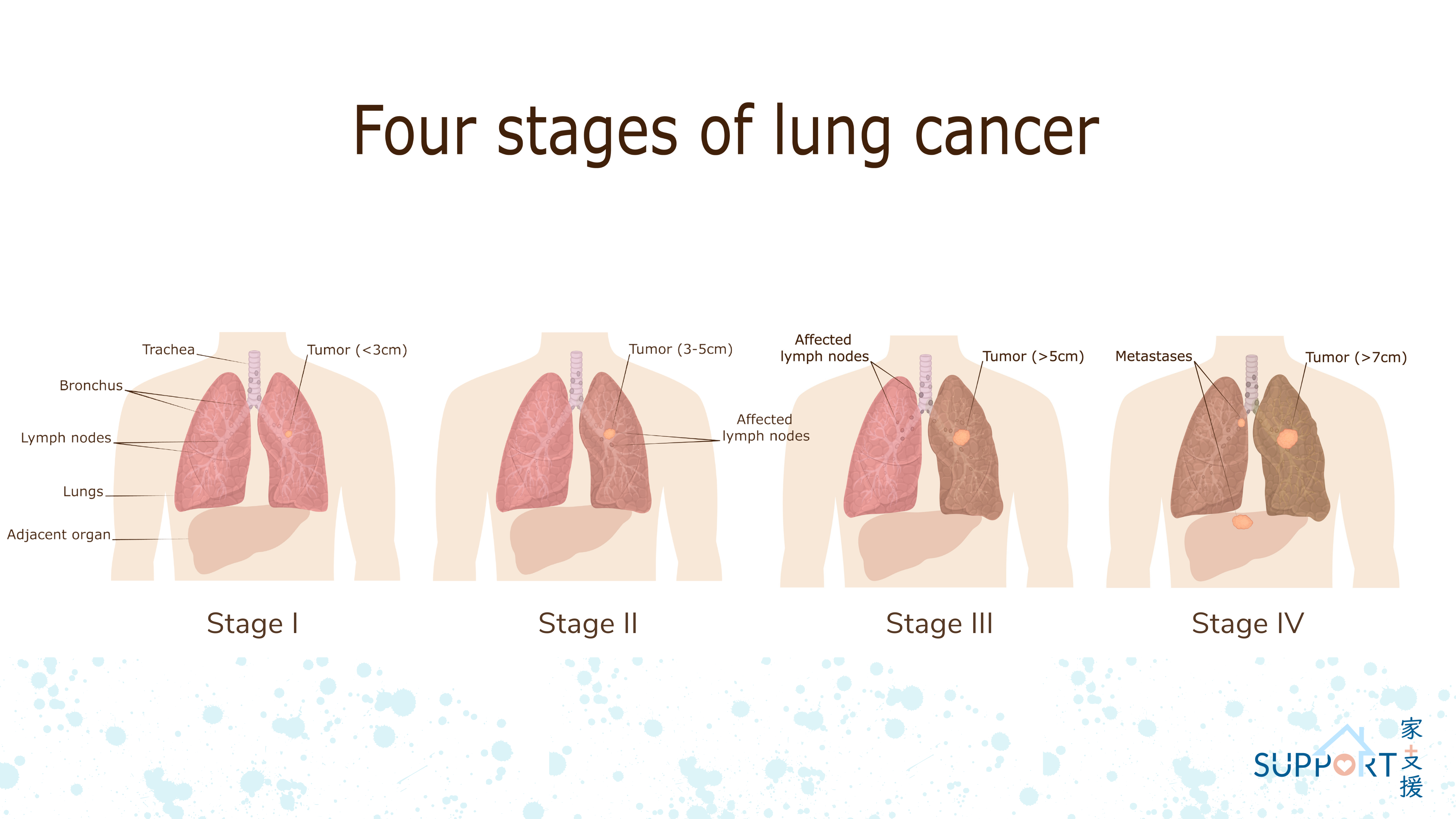
Lung cancer is a malignant tumour developed in the lower part of the respiratory system including cells in the tracheal, bronchial and bronchiolar wall.