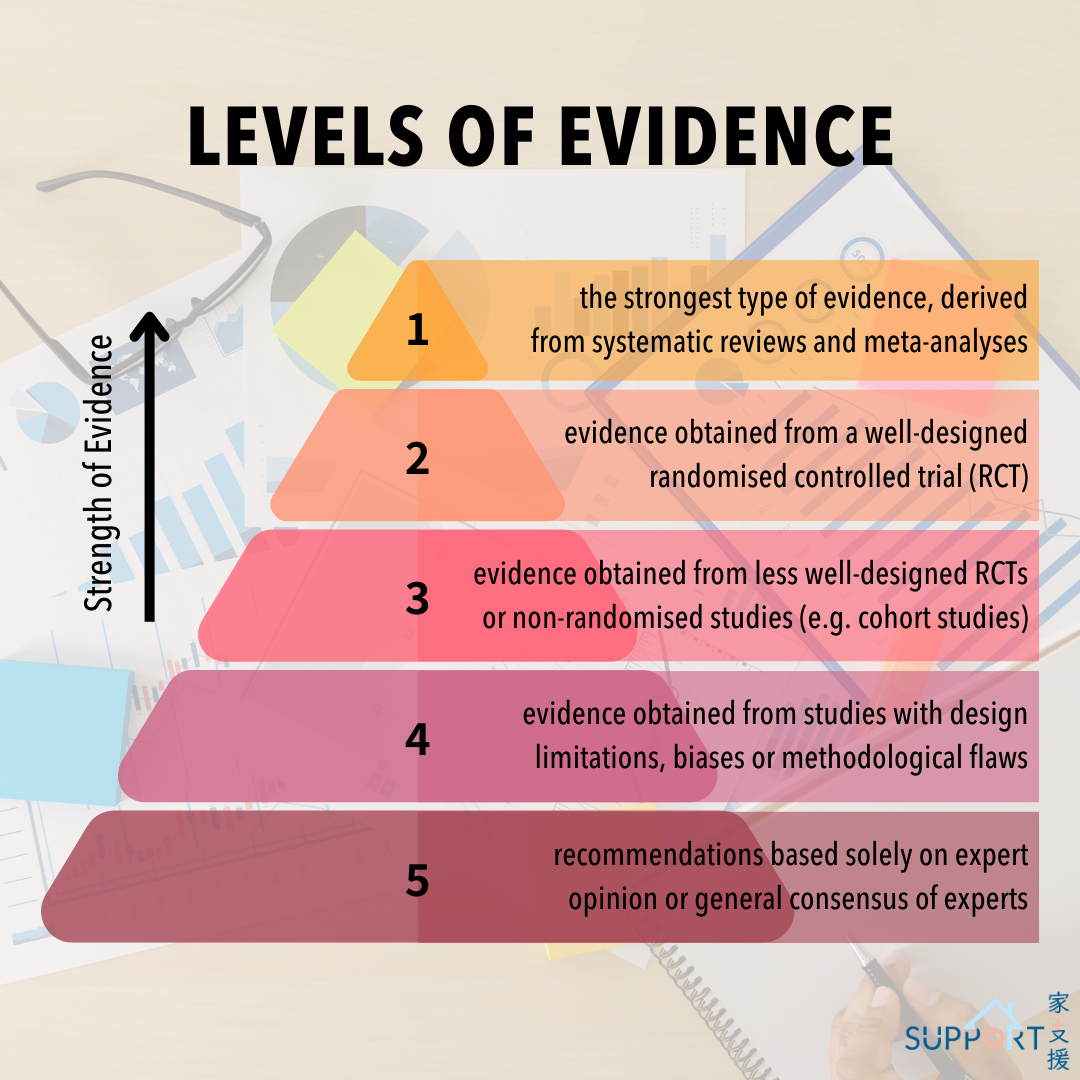
Level 1 evidence: High quality evidence
- It is the strongest type of evidence, derived from systematic reviews and meta-analyses of randomised control trials (RCTs) or current clinical practice guidelines.
- Experts analyse and evaluate many research studies using rigorous statistical methods to answer a specific clinical question about medical interventions, diagnostic tests or disease prognosis.
Level 2 evidence: Moderate quality evidence
- It refers to evidence obtained from a well-designed RCT.
- RCTs are the gold standard in clinical research which provides the most reliable type of evidence.
- The randomisation process of treatment groups (receiving the intervention of interest) and control groups (not receiving the intervention of interest) helps eliminate biases to arrive at objective and reliable results.
Level 3 evidence: Moderate-to-low quality evidence
- Evidence is obtained from less well-designed RCTs or non-randomised studies, for example, cohort studies or case-control studies.
- Cohort studies follow cohorts of patients over time to evaluate their differences in outcomes.
- Case-control studies compare patients with a specific outcome or condition with those that do not. They look retrospectively to find differences in risks or exposures between the two groups.
- Since both cohort and case-control studies are not randomised, the evidence is considered less reliable when compared with RCTs.
Level 4 evidence: Low-quality evidence
- Level 4 evidence comes from studies with design limitations, biases, or methodological flaws.
- These evidences are considered less reliable to guide clinical practices and decision-making.
Level 5 evidence: Very low-quality evidence
- Level 5 evidence is based on recommendations based solely on expert opinion or general consensus of experts in the field.
- It is subjective and may lack evidence to back up such opinions.
- It is only used when higher levels of evidence are unavailable or not applicable.
Special thanks to Ms. Yan-Tung Ho (Class M26), medical student of Li Ka Shing Faculty of Medicine, the University of Hong Kong, and Dr. Wendy Wing-Lok Chan, Department of Clinical Oncology, the University of Hong Kong, for authoring and editing this article.


